What is the Lewis Structure for NH₄⁺?
Chính Sách Vận Chuyển Và Đổi Trả Hàng
Miễn phí vận chuyển mọi đơn hàng từ 500K
- Phí ship mặc trong nước 50K
- Thời gian nhận hàng 2-3 ngày trong tuần
- Giao hàng hỏa tốc trong 24h
- Hoàn trả hàng trong 30 ngày nếu không hài lòng
Mô tả sản phẩm
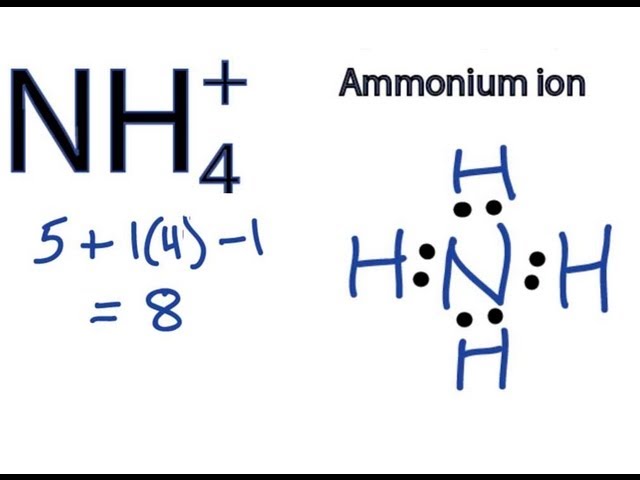
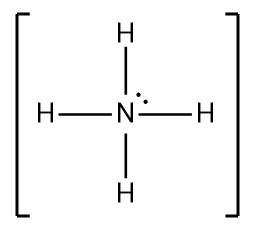
The Lewis structure for NH₄⁺ shows nitrogen (N) at the center, bonded to four hydrogen (H) atoms. Nitrogen has a positive formal charge.
Understanding the Lewis Structure of NH₄⁺
Step-by-Step Construction
1. **Count valence electrons:** Nitrogen has 5 valence electrons, and each hydrogen has 1. Since the ion has a +1 charge, we subtract one electron. Total valence electrons: 5 + (4 × 1) - 1 = 8 electrons.
2. **Central atom:** Nitrogen is the least electronegative atom and becomes the central atom.
3. **Single bonds:** Place single bonds between the nitrogen atom and each of the four hydrogen atoms. This uses 8 electrons (4 bonds × 2 electrons/bond).
4. **Formal charges:** Nitrogen starts with 5 valence electrons, it has 4 bonds (4 electrons), and no lone pairs, therefore its formal charge is 5 - 4 = +1. Each hydrogen atom has 1 bond and 0 lone pairs which results in a formal charge of 0.
5. **Final Structure:** The Lewis structure shows nitrogen in the center with four single bonds to four hydrogen atoms and a positive charge on the nitrogen atom. There are no lone pairs on the nitrogen atom. This structure fulfills the octet rule for nitrogen (8 electrons in its valence shell) and the duet rule for each hydrogen atom (2 electrons in its valence shell).
Important Considerations
The positive charge on the ammonium ion (NH₄⁺) is delocalized across the entire molecule, meaning it is not solely on the nitrogen atom, but spread across all five atoms. The Lewis structure is a simplified representation that helps visualize the bonding. The actual distribution of electron density is more complex and is better described by molecular orbital theory.
Understanding the Lewis structure is crucial for predicting the shape and properties of the ammonium ion. Its tetrahedral geometry and positive charge influence its reactivity and interactions with other molecules.
Sản phẩm liên quan: 1 năm có bao nhiêu tuần 2022
Xem thêm: câu khiến la gì lớp 3
Xem thêm: vỡ gương có đen không
Sản phẩm liên quan: sẽ gầy nghĩa đen là gì