NH₄¹⁺ Lewis Structure
Chính Sách Vận Chuyển Và Đổi Trả Hàng
Miễn phí vận chuyển mọi đơn hàng từ 500K
- Phí ship mặc trong nước 50K
- Thời gian nhận hàng 2-3 ngày trong tuần
- Giao hàng hỏa tốc trong 24h
- Hoàn trả hàng trong 30 ngày nếu không hài lòng
Mô tả sản phẩm
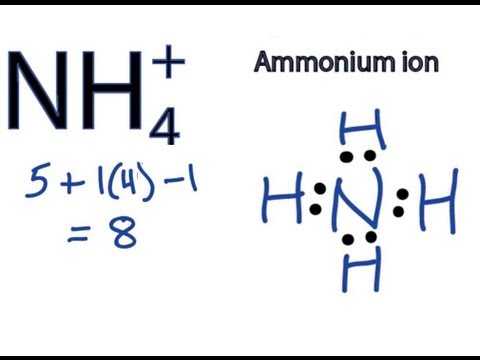
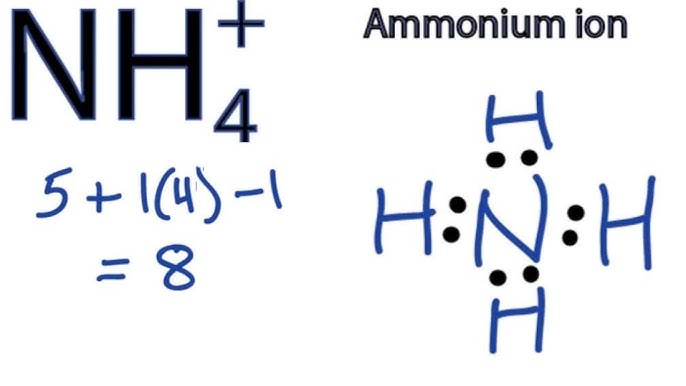
The NH₄¹⁺ Lewis structure shows the ammonium ion, a positively charged polyatomic ion. It's formed when a nitrogen atom bonds with four hydrogen atoms, resulting in a tetrahedral geometry. The positive charge indicates that the ion has lost one electron.
Drawing the NH₄¹⁺ Lewis Structure
Step 1: Count Valence Electrons
Nitrogen (N) has 5 valence electrons, and each hydrogen (H) atom has 1 valence electron. Since it's a cation with a +1 charge, we subtract 1 electron. Therefore, the total number of valence electrons is 5 + (4 × 1) - 1 = 8.
Step 2: Determine the Central Atom
Nitrogen is the least electronegative atom and will be the central atom.
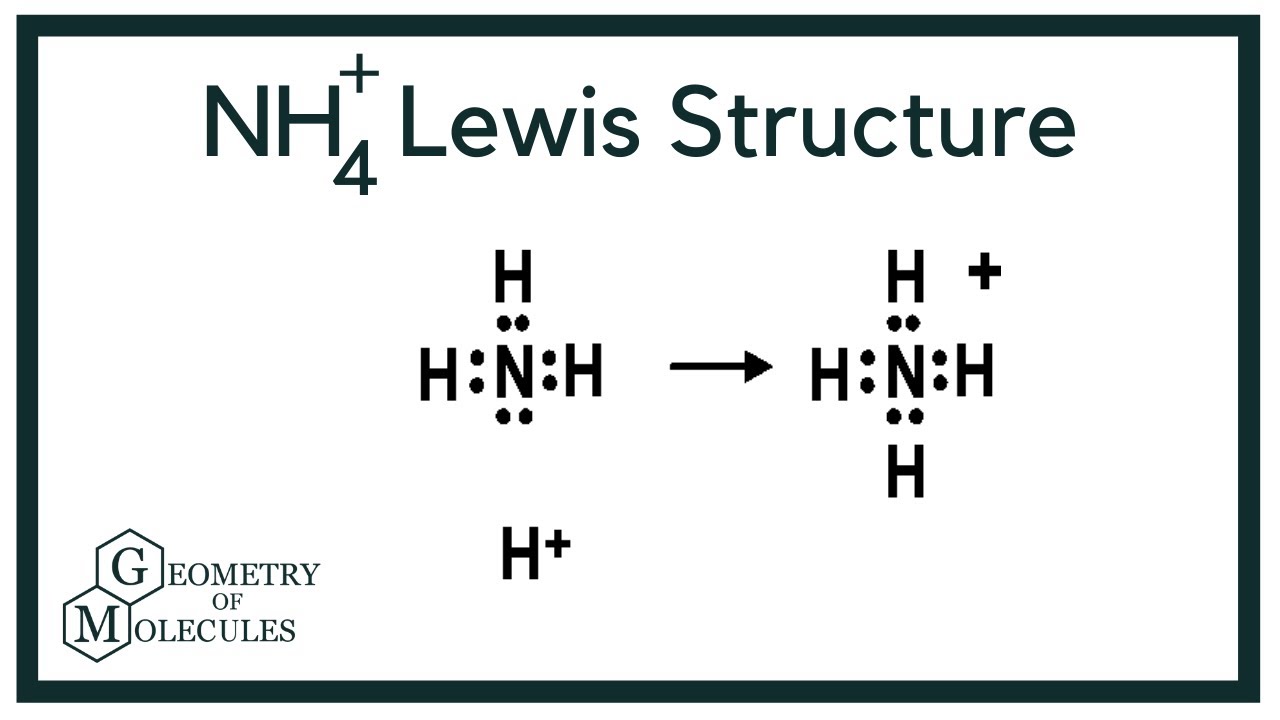
Step 3: Connect Atoms with Single Bonds
Connect each hydrogen atom to the nitrogen atom with a single bond. This uses 4 of the 8 valence electrons (4 bonds × 2 electrons/bond).
Step 4: Distribute Remaining Electrons
We have 4 valence electrons left (8 - 4 = 4). Since each hydrogen atom already has a full valence shell (2 electrons), these remaining electrons are not placed on the hydrogen atoms. The nitrogen atom is the only atom which can accept these electrons to achieve an octet.
Step 5: Check Octet Rule
The nitrogen atom now has 8 electrons around it (4 from the bonds and 4 as a lone pair), satisfying the octet rule. Each hydrogen atom has 2 electrons, satisfying the duet rule.
Step 6: Formal Charges
While we add lone pairs to complete the octet of nitrogen in a neutral molecule of NH3, this isn't the case in NH4+. The nitrogen atom has 4 covalent bonds and no lone pairs. Since it only has 5 valence electrons and 4 bonds, it has a formal charge of +1, equal to the overall charge of the ion.
Step 7: Final Structure
The final NH₄¹⁺ Lewis structure shows the nitrogen atom in the center bonded to four hydrogen atoms with single bonds. There are no lone pairs on the nitrogen atom; instead, there is a positive charge on nitrogen to reflect the formal charge of the ion. This structure accurately reflects the tetrahedral geometry of the ammonium ion.
Key Features of the NH₄¹⁺ Lewis Structure
The key features are the tetrahedral shape, the positive charge located on the nitrogen atom and that the nitrogen atom forms four covalent single bonds with four hydrogen atoms and has no lone pairs. Understanding the Lewis structure helps predict the reactivity and properties of the ammonium ion.
Sản phẩm liên quan: phan biet tieng anh
Xem thêm: bà nà hill đà nẵng
Xem thêm: hcl naalo2 hiện tượng