Lewis Structure of SO₄²⁻
Chính Sách Vận Chuyển Và Đổi Trả Hàng
Miễn phí vận chuyển mọi đơn hàng từ 500K
- Phí ship mặc trong nước 50K
- Thời gian nhận hàng 2-3 ngày trong tuần
- Giao hàng hỏa tốc trong 24h
- Hoàn trả hàng trong 30 ngày nếu không hài lòng
Mô tả sản phẩm
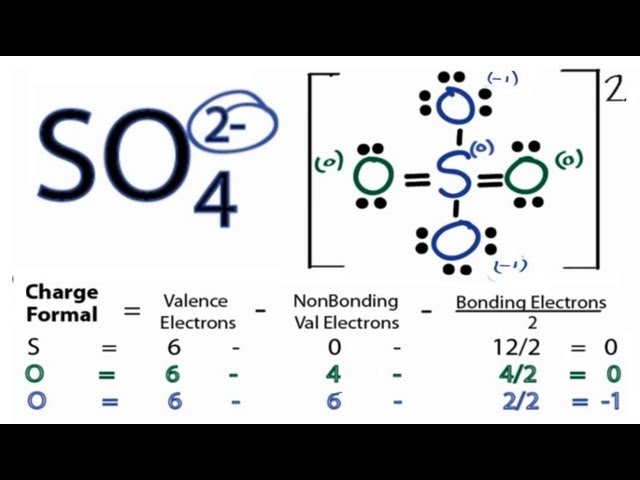
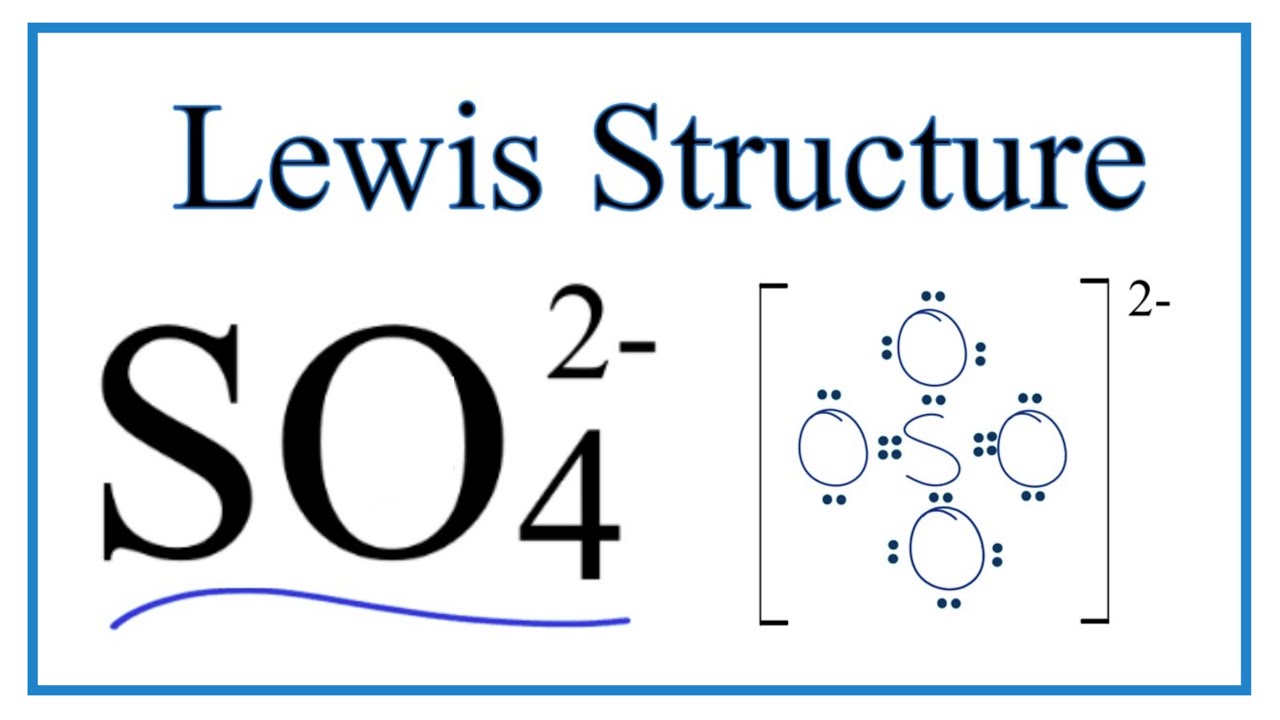
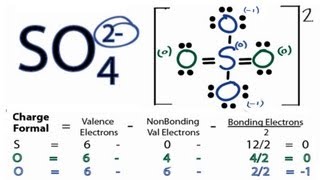
The Lewis structure of SO₄²⁻ shows a central sulfur atom surrounded by four oxygen atoms. Two oxygen atoms are singly bonded to sulfur and carry a formal negative charge, while the remaining two oxygen atoms are doubly bonded to sulfur. This arrangement minimizes formal charges and satisfies the octet rule for all atoms.
Drawing the Lewis Structure of SO₄²⁻
Step 1: Count Valence Electrons
To begin, we count the total number of valence electrons. Sulfur has 6 valence electrons, each oxygen atom has 6, and we add two more electrons for the 2- charge, resulting in a total of 6 + (4 × 6) + 2 = 32 valence electrons.Step 2: Arrange Atoms and Form Single Bonds
Place the sulfur atom in the center, surrounded by the four oxygen atoms. Connect each oxygen atom to the sulfur atom with a single bond, using 8 electrons (4 bonds x 2 electrons/bond).Step 3: Distribute Remaining Electrons
We have 32 - 8 = 24 electrons remaining. Distribute these electrons to satisfy the octet rule for each oxygen atom. This requires placing 6 electrons (3 lone pairs) around each oxygen atom, using 24 electrons.Step 4: Check Octet Rule and Formal Charges
At this point, sulfur only has 8 electrons around it, satisfying the octet rule. However, the formal charges are not minimized. Sulfur has a formal charge of +2 and two oxygens have a formal charge of -1.Step 5: Minimize Formal Charges (Resonance Structures)
To minimize formal charges, we need to form double bonds. By converting two single bonds to double bonds, we reduce the formal charges. This results in two oxygen atoms having double bonds and two oxygen atoms having single bonds. This gives us multiple resonance structures, all equally valid representations of the sulfate ion. Each resonance structure shows sulfur with a formal charge of 0, and two oxygens with a formal charge of -1. This is the most stable configuration.Step 6: Final Lewis Structure
The final Lewis structure will show the sulfur atom in the center, with two double bonds to two oxygen atoms and two single bonds to the remaining two oxygen atoms. The two singly-bonded oxygen atoms will each carry a negative charge. Remember that the actual structure is a resonance hybrid, a combination of these resonance structures.Formal Charges Calculation
Formal charge is calculated using the formula: Formal Charge = Valence Electrons - (Non-bonding Electrons + 1/2 Bonding Electrons). By calculating the formal charge of each atom in the various steps, it helps guide us towards the most stable Lewis structure.Xem thêm: tác dụng nước vối
Xem thêm: chia số thập với số tự nhiên
Sản phẩm hữu ích: ngân hàng vietcombank làm việc tới mấy giờ
Sản phẩm liên quan: nhân số thập phân
Sản phẩm liên quan: cách rửa mực không tanh