Chemistry Terms and Definitions
Chính Sách Vận Chuyển Và Đổi Trả Hàng
Miễn phí vận chuyển mọi đơn hàng từ 500K
- Phí ship mặc trong nước 50K
- Thời gian nhận hàng 2-3 ngày trong tuần
- Giao hàng hỏa tốc trong 24h
- Hoàn trả hàng trong 30 ngày nếu không hài lòng
Mô tả sản phẩm
Looking for a comprehensive list of chemistry terms and definitions? This article provides an overview of key concepts and terminology in chemistry, covering various branches of the field. We will explore definitions, explain concepts, and offer examples to help you build a strong understanding of chemical principles.
Basic Chemistry Terms
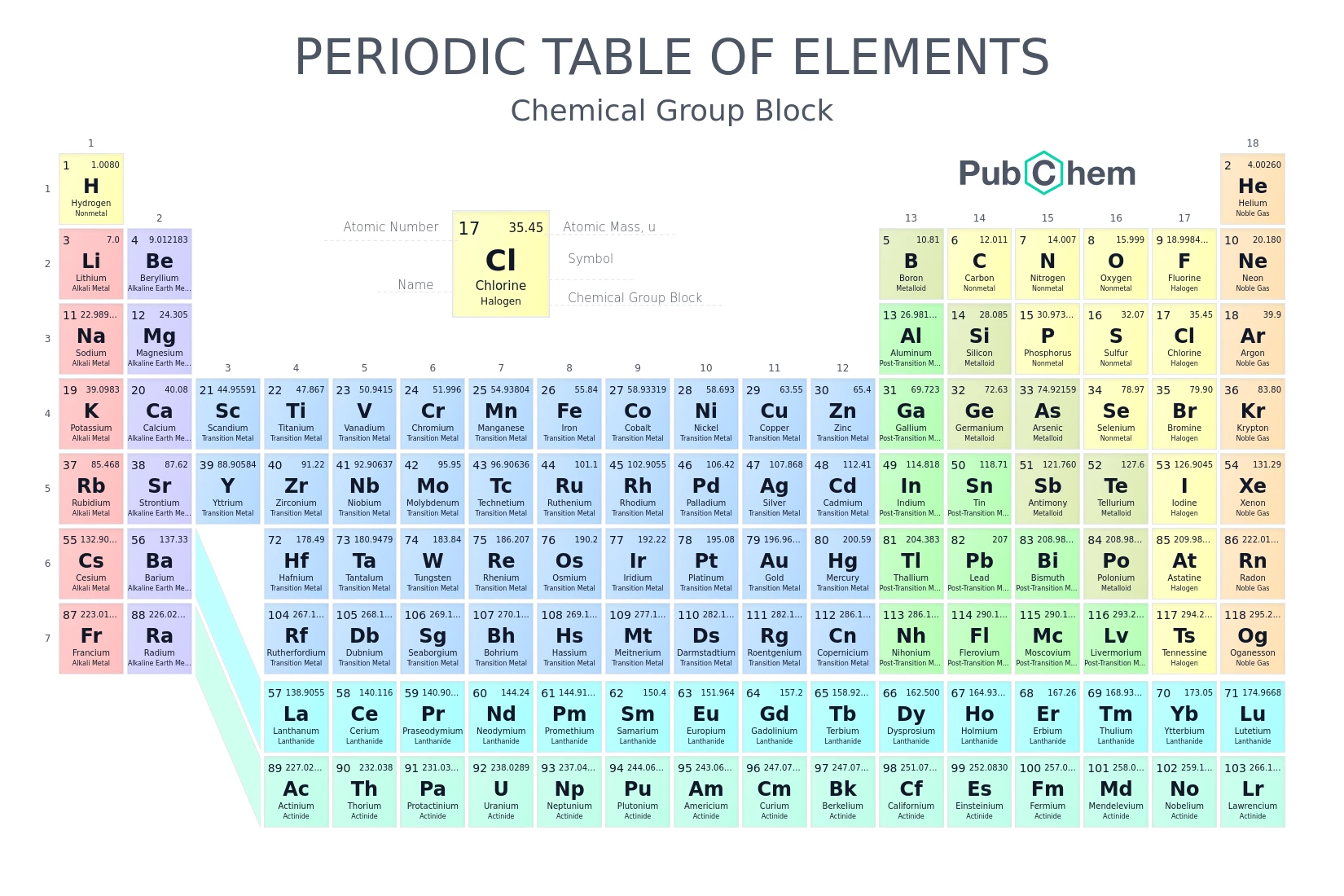
Atom:
The basic unit of a chemical element. Atoms consist of a nucleus (containing protons and neutrons) and orbiting electrons. For example, a hydrogen atom has one proton and one electron.Molecule:
A group of two or more atoms held together by chemical bonds. Water (H₂O) is a molecule composed of two hydrogen atoms and one oxygen atom.Element:
A pure substance consisting only of atoms that all have the same number of protons in their atomic nuclei. Examples include hydrogen (H), oxygen (O), and iron (Fe).Chemical Reactions
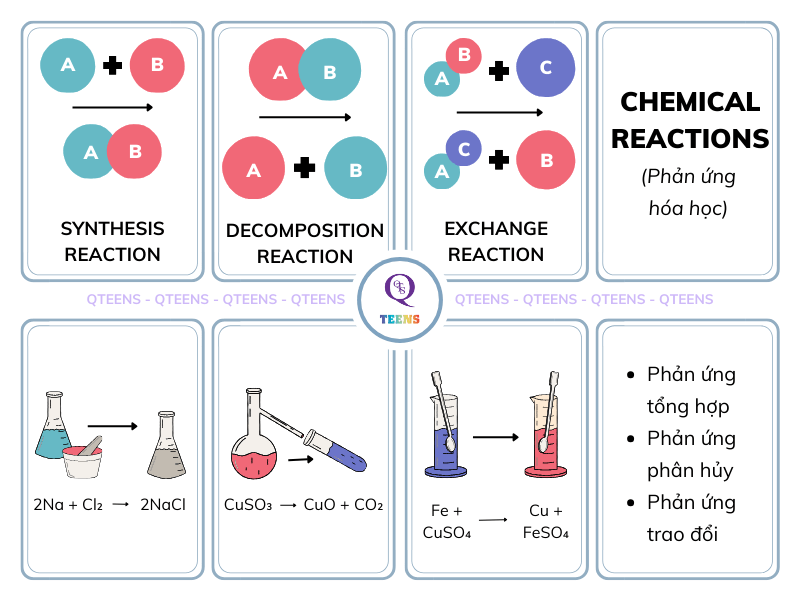
Chemical Reaction:
A process that leads to the transformation of one set of chemical substances to another. Chemical reactions involve the breaking and formation of chemical bonds.Reactant:
A substance that is present at the beginning of a chemical reaction. These substances are transformed into products.Product:
A substance that is formed as the result of a chemical reaction.Branches of Chemistry
Organic Chemistry:
The study of carbon-containing compounds and their properties. It encompasses a vast array of molecules, from simple hydrocarbons to complex biomolecules.Inorganic Chemistry:
The study of the properties and behavior of inorganic compounds, which are typically compounds that do not contain carbon-hydrogen bonds.Physical Chemistry:
The branch of chemistry that applies physics to the study of chemical systems. It explores the relationship between macroscopic and microscopic properties of matter and energy transfer in chemical processes. This is just a starting point. There are many more specific terms and concepts within each branch of chemistry. Further research into specific areas will expand your knowledge significantly. Remember to consult reputable sources such as textbooks and peer-reviewed journals for in-depth information.Xem thêm: 1000 từ là bao nhiêu trang word
Sản phẩm hữu ích: kẽm gluconat cho trẻ em
Xem thêm: các gốc axit mạnh
Sản phẩm liên quan: caoh2 ra clorua vôi