The Periodic Table of Elements
Chính Sách Vận Chuyển Và Đổi Trả Hàng
Miễn phí vận chuyển mọi đơn hàng từ 500K
- Phí ship mặc trong nước 50K
- Thời gian nhận hàng 2-3 ngày trong tuần
- Giao hàng hỏa tốc trong 24h
- Hoàn trả hàng trong 30 ngày nếu không hài lòng
Mô tả sản phẩm
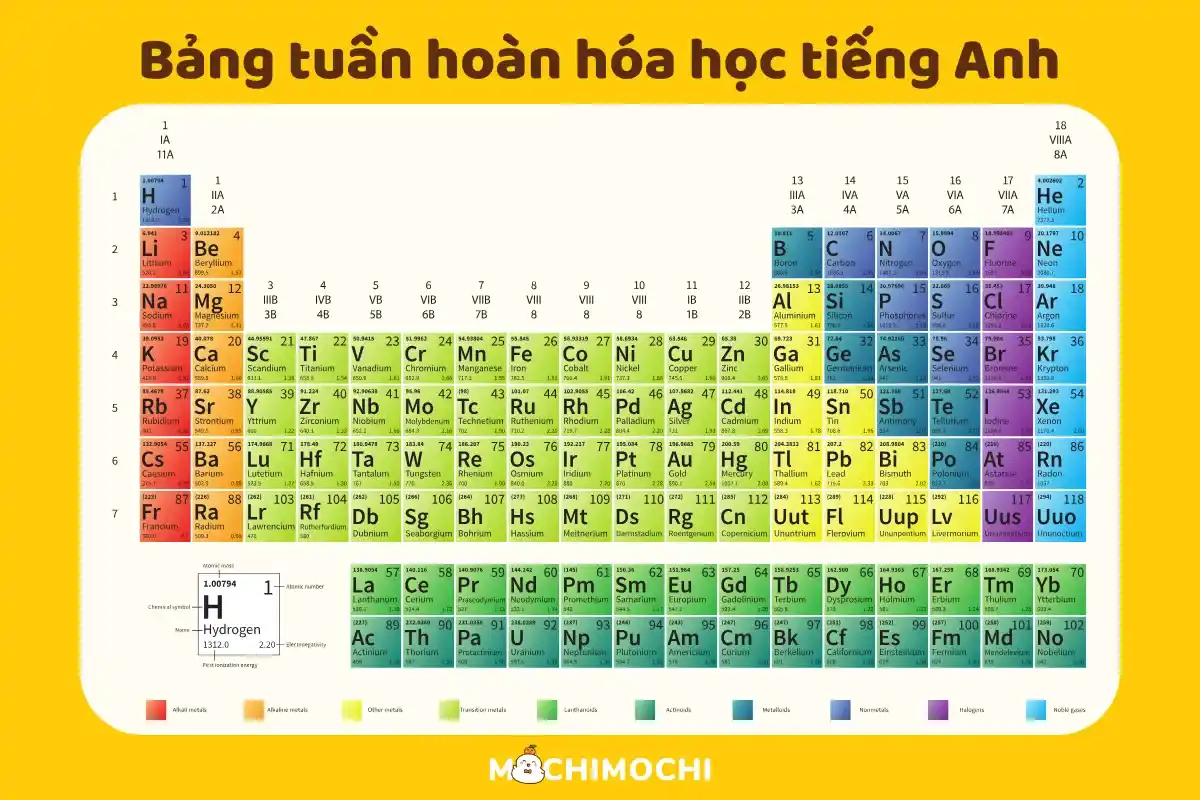
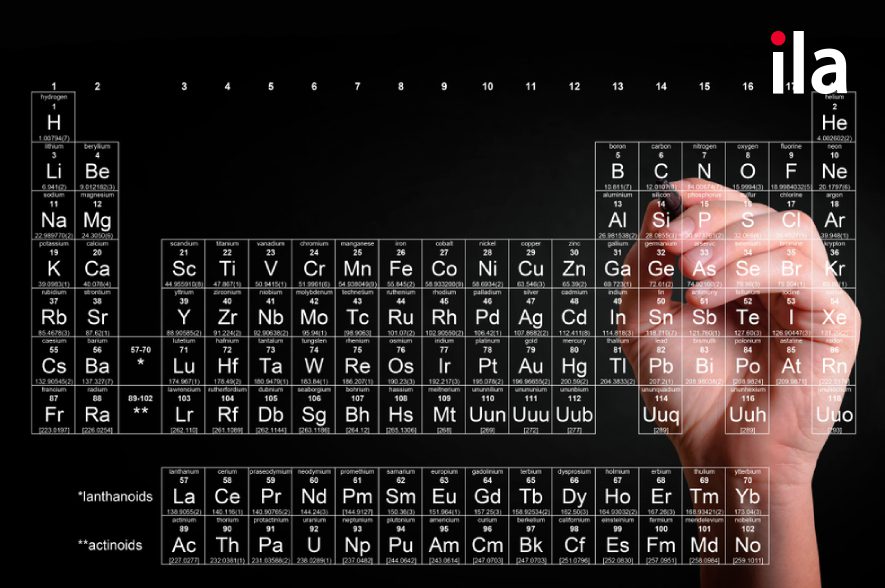
The periodic table is a tabular arrangement of the chemical elements, organized on the basis of their atomic number (number of protons), electron configurations, and recurring chemical properties. It's a fundamental tool in chemistry, providing a visual representation of the relationships between different elements.
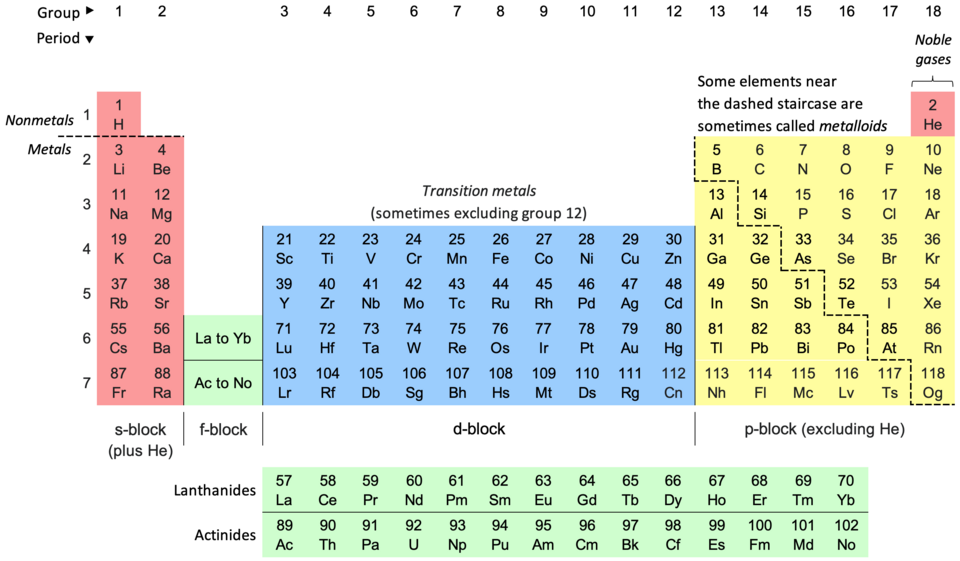
Understanding the Organization
Atomic Number and Arrangement:
The elements are arranged in order of increasing atomic number, which dictates the number of protons in the atom's nucleus. This arrangement leads to the periodic repetition of similar chemical properties, as elements with similar electron configurations tend to behave similarly.Periods and Groups:
The table is organized into rows called periods and columns called groups (or families). Elements within the same group share similar chemical properties due to having the same number of valence electrons (electrons in the outermost shell). Elements in the same period have the same number of electron shells.Element Information:
Each element's box typically contains its symbol (e.g., H for hydrogen, O for oxygen), atomic number, and atomic mass. Sometimes, other information like electron configuration or electronegativity may also be included.Key Uses of the Periodic Table
Predicting Chemical Properties:
The periodic table allows chemists to predict the properties of elements based on their position in the table. For example, elements in Group 1 (alkali metals) are highly reactive, while elements in Group 18 (noble gases) are generally inert.Understanding Chemical Reactions:
The table helps understand how elements react with each other. Elements with similar electronegativity tend to form similar types of compounds.Applications in Various Fields:
The periodic table is essential across various scientific disciplines, including chemistry, physics, materials science, and engineering. It is used to design new materials, understand chemical processes, and develop new technologies.Xem thêm: đề kiểm tra tiếng việt lớp 2 kết nối tri thức
Sản phẩm hữu ích: dòng điện cảm ứng là gì
Xem thêm: tranh vẽ chùa 1 cột
Sản phẩm liên quan: đổi km/h ra m/s